ARTICLES BY MATTHEW PILLAR
-
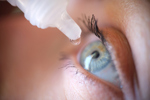
Eying Up The Era Of Topical Biologics3/19/2024
Claris Bio’s phase 1/2 clinical trial in patients with Stage 2 or 3 Neurotrophic Keratitis (NK) is breaking new ground in the development of topically administered biologic therapies.
-
Topical Biologics In Ophthalmology?3/21/2023
A recent clinical study of an eyedrop formulated with Grifols’ Immunoglobulin-based Flebogamma DIF offers cause for optimism among multiple stakeholders. It could become the first-approved topically administered biologic therapy for dry eye disease.
-
Gene Cargos: Building A Bigger Ship5/6/2022
Dissatisfied with the limitations of AAV gene editing, Drs. Ray Tabibiazar and Joe Higgins launched SalioGen, a company with an ambitious goal: the safe delivery of large strands of DNA cargo that mimics the way it happens in nature.
-
Ocular + Auditory Cell Therapies: Lineage Cell Therapeutics 2.04/25/2022
On the heels of a lucrative deal with Roche/Genentech for its Phase I/IIa dry age-related macular degeneration candidate, Lineage Cell Therapeutics is fording new waters with a promising auditory neuropathy spectrum disorder candidate. CEO Brian Culley shares the strategy.