INHALATION DRUG DELIVERY ARTICLES
 GenAI: The Muscle Behind Strong Regulatory Intelligence For Combination Products
GenAI: The Muscle Behind Strong Regulatory Intelligence For Combination Products
In this article, combination product consultant Doug Mead provides the rationale and offers guidance for using GenAI tools to search regulatory databases. He shows the benefits for biopharma developers of conducting “precedent research” into previous regulatory pathways and results for similar or related drug delivery product submissions.
INHALATION DRUG DELIVERY RESOURCES
-
This guide shares the steps involved in formulation development, considerations during formulation, and how partnering with an experienced CDMO can lead to a successful commercial product.
-
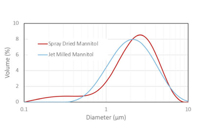
Explore the material and performance properties of spray-dried and jet-milled mannitol for respiratory delivery of crystalline mannitol.
-
Combination therapies delivered directly to the lung by dry powder inhalants are an effective means of reducing patient burden. Read about case studies that illustrate the potential for improving care.
-
Explore an integrated service package designed to simplify and accelerate the development pathway, even for APIs with challenging properties such as low aqueous solubility and poor bioavailability.
-
The $118 billion combination-product market is projected to increase at a CAGR of 8.8%. This article answers the question: “Is this actually a combination product? What do we need to do if it is?”
-
Discover how patient-centric design principles can improve the development of medical devices. By focusing on user needs and behaviors, manufacturers can ensure that products align with expectations.
-
Review a process where the insoluble and unstable free-base form of clopidogrel is converted to a form with acceptable drug loading and is protected from chemical degradation.
INHALATION DRUG DELIVERY SOLUTIONS
-
A focused look at how low‑GWP propellant technologies support sustainability goals while preserving inhaler performance, offering guidance for planning regulatory readiness, device compatibility, and long‑term respiratory portfolio strategy.
-
Our formulation development and material sciences experts have over 30 years’ experience in pre-formulation and solid state characterization.
-
Kymanox provides turnkey services to bring your product from concept to commercialization — and helps keep your product on the market. Kymanox has expertise in injectables (e.g., syringes, mechanical and electromechanical autoinjectors, wearable injectors, dual chamber systems, reconstitution systems), respiratory combination products (e.g., metered dose inhalers, dry powder inhalers, nasal sprays), and in ocular products (e.g., multi-dose containers, single-use injectables).
-
By partnering with Battelle, your organization can reduce risks and save on investment costs, leading to more successful and sustainable outcomes.
-
Integrated device assembly, labeling, and packaging solutions streamline pharma delivery, ensuring compliance, scalability, and patient-centric design from clinical trials to commercial production.
-
Our newly expanded in-house capabilities span clinical and commercial scale manufacturing for DPIs, unit-dose, bi-dose and preserved multi-dose nasal sprays.
-
Learn how our team of scientists, engineers, and human-centered designers, as well as our world-class facilities, empower us to confidently guide your product toward a successful market launch.
-
Modern inhalation platforms, improved formulations, and greener propellants speed development and strengthen respiratory performance, with key factors guiding device choice and clinical readiness.
-
An overview of analytical capabilities to boost product quality, meet regulatory expectations, and drive development through phase‑appropriate methods and specialized testing for complex drug programs.
-
Explore new approach methodologies, integrating in vitro and computational models to improve toxicology testing, and drug development, enhancing accuracy, efficiency, and human relevance.