TRANSDERMAL DRUG DELIVERY ARTICLES
 Conducting A Medical Device Stability Study: A Practical Guide
Conducting A Medical Device Stability Study: A Practical Guide
Stability studies measure how medical devices respond to environmental stresses such as temperature, humidity, light, and time. Let's take a closer look at designing and conducting a stability study.
TRANSDERMAL DRUG DELIVERY RESOURCES
-
Screen-Printed Innovative Drug Technology can produce oral, transdermal, and implantable dosage forms while ensuring heterogeneous distribution of active ingredients.
-
Explore the rationale behind opting for a contract development organization (CDO) to fulfill organizational requirements and underscore vital considerations for evaluating CDOs to ensure alignment with your project objectives.
-
Drug delivery is one of the most significant obstacles to advancing gene therapies. Examine the opportunities for success in combining novel medical devices to improve delivery of gene therapies.
-
Innovative approaches in developing formulations can help to improve a drug's safety and efficacy. Explore the decision for selecting the best-suited technology for formulating a complex molecule.
-
Microphysiological systems (MPS) use human cells to model tissue function, offering faster, more predictive insights for drug discovery, safety testing, and personalized medicine—bridging gaps left by animal and 2D models.
-
This guide shares the steps involved in formulation development, considerations during formulation, and how partnering with an experienced CDMO can lead to a successful commercial product.
-
The $118 billion combination-product market is projected to increase at a CAGR of 8.8%. This article answers the question: “Is this actually a combination product? What do we need to do if it is?”
TRANSDERMAL DRUG DELIVERY SOLUTIONS
-
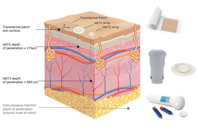
We know what it takes to deliver drug products to and through the skin. Explore drug-in-adhesive patches, solid microneedles (sMTS), and hollow microneedles (hMTS).
-
This comprehensive suite of services encompasses the entire spectrum of user research, starting from the conceptualization of study designs to meticulous data analysis and comprehensive reporting.
-
Discover the nanoparticle engineering, formulation and GMP manufacturing services that can drive forward your market success and unlock the power of “small."