INFUSION DRUG DELIVERY ARTICLES
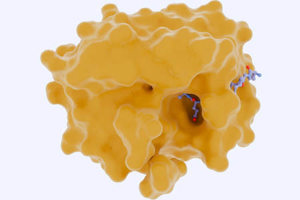
 The Future Of Kidney Disease Therapeutics: Bringing Nanomedicine To Nephrology
The Future Of Kidney Disease Therapeutics: Bringing Nanomedicine To Nephrology
Nanomedicines are solving the drug delivery problem in kidney disease. The next step: bridging the translational gap to advance these technologies into the clinic.
INFUSION DRUG DELIVERY RESOURCES
-
Healthcare is shifting homeward. With telehealth, connected devices, and user-friendly design, patients and caregivers now deliver hospital-level care safely and effectively outside clinical settings.
-
Explore the reasons why one CDMO is expanding its operations to offer flexible bag filling, after being equipped with filling lines for PFS and glass vials (glass or plastic).
-
Everything from glass or plastic bottles to the ink used in labels can leach unwanted contaminants. The first challenge chemists address is to narrow the focus on the most likely suspects.
-
The $118 billion combination-product market is projected to increase at a CAGR of 8.8%. This article answers the question: “Is this actually a combination product? What do we need to do if it is?”
-
Early stage formulation studies, which should support the emerging target product profile, will often yield important experimental results to aid subsequent development of clinically relevant dosage forms.
-
Combination products provide significant avenues for increasing patient adherence and overall efficacy. This collection outlines the opportunity and identifies strategies for gaining regulatory approval.
-
Learn what you need to know about planning for your extractables and leachables testing programs to mitigate delays to your drug development timelines.
INFUSION DRUG DELIVERY SOLUTIONS
-
By partnering with Battelle, your organization can reduce risks and save on investment costs, leading to more successful and sustainable outcomes.
-
This comprehensive suite of services encompasses the entire spectrum of user research, starting from the conceptualization of study designs to meticulous data analysis and comprehensive reporting.
-
In recent years, hospitals, clinics, and others have experienced chronic shortages of medical solutions. The Solutions for Life initiative is a billion-dollar investment in meeting nationwide demand for these products.
-
Learn how our team of scientists, engineers, and human-centered designers, as well as our world-class facilities, empower us to confidently guide your product toward a successful market launch.
-
Examine a NVGD platform that tackles the primary obstacle hindering gene editing therapies: efficient delivery. Utilizing engineered nanoparticles, the platform overcomes limitations associated with payload size.
-
RASR filling and closing machines are designed to treat IV bags. These machines are able to fill single chamber as well as dialysis multi-chambers bags.
-
Capabilities that improve efficiency and accelerate development.
B. Braun's OEM Division offers a variety of in-house molding capabilities including injection molding, insert molding and over molding. They own a primary 400,000-square-foot U.S. plant that includes a 16,500-square-foot ISO Class 8 molding facility housing some of B. Braun’s 80 injection molding presses, which range from 55-330 tons.
-
BD Neopak™ XtraFlow™ Glass Prefillable Syringe solution leverages 8 mm needle length in combination with thin wall cannula to reduce pressure drop and enhance flow.
-
These containers can be delivered with a full line of solutions used in clinical settings, including basic saline, heparin premix, and lidocaine. The chemistry used to produce the container uses neither polyvinyl chloride (PVC) nor the plasticizer DEHP.
INFUSION DRUG DELIVERY NEWS
- Melbourne-Developed Drug Delivery Platform Delivers 'Impressive' Results In International Bowel And Ovarian Cancer Trial
- Race Executes Agreement With Ardena For GMP Manufacturing Of RC220
- Acasti Announces WuXi Clinical As CRO To Conduct STRIVE-ON Pivotal Phase 3 Safety Trial For GTX-104 In aSAH Patients
- Ascendia Pharmaceuticals Develops Novel Nanoemulsion IV Formulation For Clopidogrel That Received IND Approval
- Viridian Therapeutics Announces Partnership With Drug Delivery Innovator Enable Injections
- Scaling Up Capacity, Eitan Medical Opens New Manufacturing Facility Bolstered by Semi-Automated Production Lines