Enteric Coatings For Oral Solid Dosages: A Short Guide
When a drug is consumed orally, in most cases the drug/API needs to be delivered into the small intestine. However, the drug needs to pass through the acidic stomach environment before getting to the intestine. Enteric coatings play a pivotal role in modern pharmaceuticals, enabling the effective delivery of medications that would otherwise be compromised by the harsh conditions of the stomach. Their protective barrier ensures that medications reach the small intestine intact, optimizing their therapeutic efficacy.
Mechanism Of Action
Enteric coating polymers have pH-dependent solubility as an inherent property. Coatings made with such polymers are resistant to gastric acid but can be readily dissolved in the alkaline environment of the small intestine. The stomach’s gastric environment has pH~3, whereas the pH at the intestine can range from pH 7 to pH 9.
When a coated medication is ingested, it travels down the esophagus and reaches the stomach. The acidic environment of the stomach triggers the enteric coating to remain intact, safeguarding the medication within. As the medication progresses into the duodenum, the pH becomes more alkaline, causing the enteric coating to dissolve. This allows the medication to be released and absorbed in the intestine, where conditions are more favorable for therapeutic action. Cellulose derivatives such as cellulose acetate phthalate (cellacefate), hydroxypropyl methylcellulose phthalate (HPMCP), and polyvinyl acetate phthalate (PVAP) are commonly used in enteric coatings.
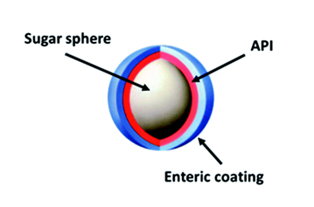
Figure 1: Schematic illustration of a digestive tract and how enteric coating works
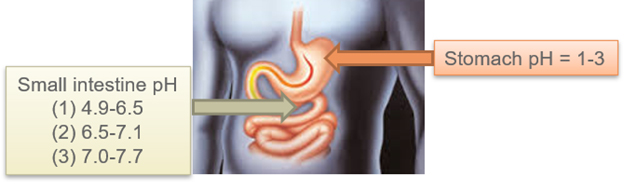
Figure 2: pH values of the small intestine and stomach
Advantages Offered By Enteric Coatings
- Gastrointestinal Protection: The primary advantage of enteric coatings is their ability to protect medications from the harsh acidic environment of the stomach. This is particularly crucial for drugs that may be inactivated or degraded by stomach acid.
- Improved Bioavailability: Enteric coatings ensure that medications are absorbed in the small intestine, where absorption is generally more efficient compared to the stomach. This leads to enhanced bioavailability and efficacy.
- Reduced Gastrointestinal Side Effects: Medications with enteric coatings are less likely to cause gastrointestinal side effects, such as nausea or gastric irritation, as they bypass the stomach.
- Enhanced Stability of Sensitive Compounds: Enteric coatings are instrumental in preserving the stability and effectiveness of medications containing sensitive or reactive compounds.
Commonly Used Enteric Coating Excipients
- Cellulose acetate phthalate
- Hydroxypropyl methyl cellulose phthalate
- Methyl acrylate-methacrylic acid copolymers
- Polyvinyl acetate phthalate (PVAP)
- Hydroxypropyl methyl cellulose acetate succinate
Applications Of Enteric Coatings
- Acid-Labile Medications: Enteric coatings are indispensable for medications that are sensitive to stomach acid. For instance, certain antibiotics, such as erythromycin, are best protected by enteric coatings to ensure their efficacy.
- Gastric Irritant Drugs: Medications known to cause irritation or damage to the stomach lining, such as nonsteroidal anti-inflammatory drugs (NSAIDs) like aspirin, benefit greatly from enteric coatings.
- Delayed Release Formulations: Enteric coatings are instrumental in creating delayed-release formulations, where the medication is released at a specific time or location in the gastrointestinal tract.
- Enzyme-Sensitive Drugs: Certain enzymes in the digestive system can rapidly break down medications. Enteric coatings help protect these drugs until they reach the small intestine where they can be absorbed effectively.
- Targeted Delivery: Enteric coatings are used in conjunction with other drug delivery technologies, such as microspheres or liposomes, to achieve targeted drug delivery to specific regions of the gastrointestinal tract.
Examples Of Marketed Enteric Coating Drugs1
- Alophen – Bisacodyl 5 mg USP (Numark Laboratories Inc.)
- Arthrotec – Diclofenac sodium and misoprostol (Pfizer)
- Azulfidine EN – Sulfasalazine
- Cardizem CD 360 mg – Diltiazem (Aventis)
- Bayer EC – Aspirin 81 mg (Bayer)
- Ery-Tab – Erythromycin 500 mg (Arbor Pharmaceuticals)
- Omez – Omeprazole (Dr. Reddy’s)
- Micropirin 75 mg EC – Aspirin 75 mg (Dexel-Pharma Ltd)
- Lofnac 100 – Diclofenac sodium 100 mg (Bliss GVS Pharma)
- Ecotrin – Aspirin 325 mg (Prestige Consumer Healthcare, Inc.
Three Commonly Reported Issues With Enteric Coatings
The use of coatings as a mechanism to modify the release of the drug is sophisticated, and some coatings have sensitivities that can lead to inconsistent product performance. When drug dissolution is not in line with expectations, typically it will be due to one of the below root causes.
- Hydrolysis: Enteric coating excipients can be sensitive to moisture and the storage conditions could lead to degradation (i.e., hydrolysis). Products coated with hydrolyzed polymers will experience inconsistent product performance. It is important to have preventive measures, such as proper storage and usage of desiccants, if needed to prevent hydrolysis of the excipient.
- Coating film cracking: Brittle films will eventually crack. Brittleness can be caused by coating shrinkage, limited flexibility, excessive thickness, or being applied/cured at too high a temperature. One standard way of reducing film brittleness that leads to cracking is to incorporate a plasticizer in the coating formulation.
- Plasticizers: A plasticizer works by providing increased motion of the polymer chains by inserting itself between the polymer chains. This provides more space for improved chain mobility. As a result, the coating becomes tougher and can better withstand stressful forces. Typical plasticizers include diethyl phthalate (DEP), glycerol triacetate (GTA) and triethyl citrate (TEC), triacetin, tripropionin, triethyl citrate, tributyl citrate, tributyl 2-acetyl citrate, and poly(ethylene glycol) of low molecular weight. Note that while there are many plasticizers used in drug manufacturing and coatings, certain plasticizers have better compatibility with certain enteric coatings’ chemistries. There needs to be trial and error for optimal formulation with the suitable plasticizer to achieve the desired flexibility without compromising on the coating.
Manufacturing Considerations
While many enteric coatings can be processed using water as a solvent, some enteric coatings require organic solvents, including acetone or ethanol. Using organic solvents in manufacturing creates additional challenges of safe handling, environmental impact, regulatory compliance, waste management, and, eventually, increased cost. Water is the preferred solvent for manufacturing, but there are APIs that are sensitive to moisture and in such cases enteric coating using organic solvents is preferred. It will be valuable if enteric coating excipients are offered for both organic solvents and aqueous processing. In many cases, the excipient does not have to be soluble in water but needs to be dispersible in water.
Future Trends And Innovations
The field of enteric coatings is continuously evolving. Researchers are exploring novel polymers and techniques to enhance the efficiency and versatility of enteric coatings. Additionally, advances in nanotechnology may lead to the development of more sophisticated, precision-engineered coatings that can deliver drugs to even more specific locations within the gastrointestinal tract.
As research and technology continue to advance, enteric coatings are poised to play an even greater role in the development of innovative drug delivery systems, ultimately benefiting patients around the world.
Reference
1. https://www.pharmapproach.com/enteric-coating-2/#:~:text=An%20enteric%20coating%2C%20also%20known,it%20reaches%20the%20small%20intestine.
About The Author:

Rajendran (Raj) Arunagiri has been in the pharma industry for a decade and has successfully developed and launched a new excipient. He is a co-author of technical articles and is an invited speaker at conferences focused on excipients and drug delivery. He specializes in the area of poorly soluble APIs and modified release. Arunagiri welcomes you to reach out to him for questions, comments, and collaboration ideas at raj.gceb@gmail.com.
