Formulation Development Of A JAK1 Inhibitor Extended-Release Tablet
By Dileep Boinipally, director of drug product formulation, Metsera

The subject small molecule drug compound (called hereinafter drug substance) is potent selective Janus kinase 1 (JAK1) inhibitor. This drug compound was chosen as a model drug to demonstrate the formulation approach because a once-daily dosing regimen is preferred for optimal therapeutic compliance, as with other approved JAK inhibitors and based on its pKa, LogP, and solubility characteristics, among others. This necessitates the need for an extended release (ER) oral solid dosage form. The drug substance’s chemical structure, potential indication, and additional intricate details of drug product design are not disclosed in this article. This scientific article is strictly to present an in-depth summary of the formulation development strategy, with a focus on experimental design, data interpretation, and formulation decision-making logic intended for first-in-human clinical studies.
Objectives And Challenges
The overarching objective was to develop a robust ER matrix tablet containing 40 mg of drug substance, with consistent 24-hour release, appropriate pharmacokinetic performance, manufacturability via direct compression, and stability. Key challenges included the drug substance’s pH-dependent solubility, variability in gastrointestinal (GI) environments, and the need to identify and control critical formulation variables.
Physicochemical Properties Of Drug Substance
Table 1 below summarizes the critical physicochemical attributes of the subject drug substance.
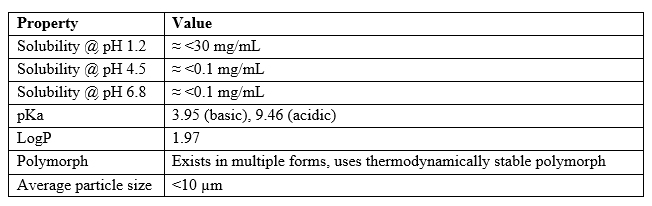
Table 1
These properties pointed to a pronounced pH-dependent solubility profile, necessitating formulation strategies to modulate microenvironmental pH and sustained drug release.1
Manufacturing Process Strategy
A direct compression process was selected to facilitate simplicity, scalability, and cost-effectiveness. The standard process sequence included:
- screening
- pre-blending drug substance with filler
- main blending with polymer, acidulants, and glidants
- lubrication
- compression (target tablet weight and hardness).
Formulation Risk Assessment: Initial Strategy
Formulation variables were categorized based on prior experience and potential impact to extended-release dissolution profile (see Table 2 below).2
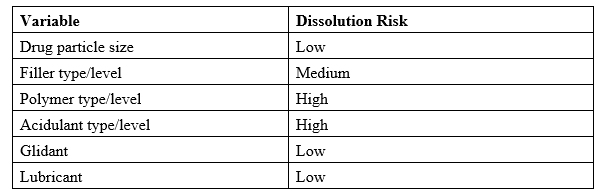
Table 2
Risk prioritization directed experimental effort toward polymers and acidulants. Fillers were studied for secondary impacts on compressibility and hydration.
Polymer Selection And Concentration Studies
A matrix-based diffusion-controlled release system was pursued. Hydroxypropyl methylcellulose (HPMC) was selected due to its well-characterized performance in ER systems.3
Table 3 below presents compositions and key findings for early HPMC prototype studies.

Table 3
Observations:
- Increased HPMC concentration led to slower release.
- The combination of HPMC grades introduced variability, possibly due to segregation.
- Batch 001 offered controlled release with acceptable linearity.
pH-Modifying Excipients (Acidulants): Experimental Rationale
Due to low solubility above pH 4.5, acidulants were studied to modulate microenvironmental pH within the matrix. Tartaric acid and fumaric acid were selected for evaluation based on their pKa (~3) compatibility with the drug substance.4, 5
Fumaric Acid Studies
Fumaric acid at 15% w/w improved release post pH shift (see Table 4 below), but a nonlinear release curve was observed due to its slow solubilization.

Table 4
Tartaric Acid Studies
Tartaric acid provided faster initial solubilization and eliminated the inflection point upon pH change (see Table 5 below). From this point on, HPMC K100 LV grade is used as a release controlling polymer for a faster and less variable dissolution profile.
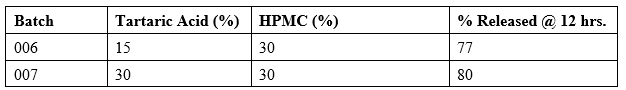
Table 5
Combined Acidulants
The synergistic use of both acidulants (10% fumaric + 20% tartaric) was optimal. Table 6 below summarizes the dissolution performance of selected formulations with varying combinations of fumaric and tartaric acid.
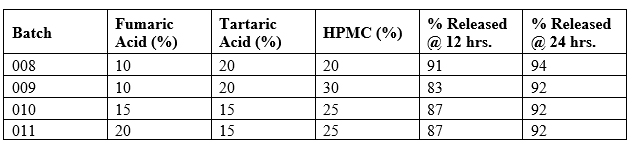
Table 6
All formulations demonstrated linear sustained release with minimal inflection during the pH transition. Notably, batch 009 with 30% HPMC and balanced acidulants showed optimal control with 83% release in 12 hours (slowest among all for ER profile) and complete release by 24 hours. This approach effectively balanced immediate pH buffering (via tartaric acid) with sustained internal acidification (via fumaric acid), resulting in predictable, pH-independent release kinetics.
Filler Evaluation
Three fillers and their combinations were studied: lactose monohydrate, microcrystalline cellulose (MCC), and dicalcium phosphate (DCP). The results are tabulated in Table 7 below. HPMC K100 LV polymer concentration was fixed at 30% w/w.
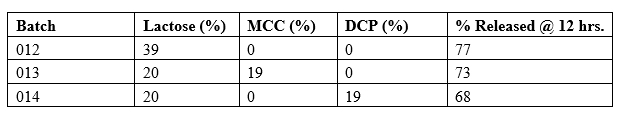
Table 7
Lactose yielded optimal hydration and erosion-based release. Insoluble fillers reduced release due to poor matrix hydration.
Tablet Size Study
Tablet weight increased from 267 mg to 333 mg to increase overall tablet thickness/surface area (a 25% increase in tablet weight), to evaluate impact on diffusion path. Larger tablets demonstrated slower release, as expected, as seen in Table 8 below. HPMC K100 LV polymer concentration was fixed at 30% w/w.

Table 8
Batch 16, with both tartaric acid and fumaric acid, was selected for human dosing, knowing the drug substance’s pH dependent solubility and the need for in vivo control on drug substance solubility and release rates. As expected, this formulation demonstrated optimal exposure and controlled Cmax. The dual acidulant strategy prolonged Tmax and improved overall PK consistency, i.e., AUC of 250 ng*hr/mL and Cmax of 39 ng/mL in line with other marketed JAK1 inhibitor extended-release formulations.6
Stability And Manufacturability
Batch 16 was stable for over one month at 25 degrees C/60% RH and 40 degrees C/75% RH, with no significant degradation or change in dissolution. Sticking and poor flow observed during compression prompted the increase of colloidal silicon dioxide from 0.5% to 1.0% for the next phase of development. A moisture-barrier film coat is recommended to reduce visual defects.
Final Formulation
Below (Table 9) is the final formulation that was the result of a systematic, risk-based formulation development approach.
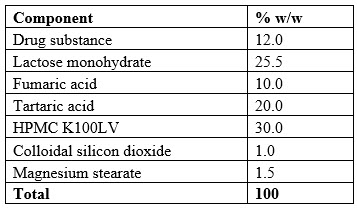
Table 9
Conclusion
This first in human oral formulation development of the subject JAK1 inhibitor drug compound was marked by a rational, data-driven approach integrating polymer science, solubility modulation, and in vivo performance. Key success factors included:
- controlled release via HPMC K100LV
- pH modulation using tartaric and fumaric acid
- lactose-based erosion matrix
- verification of extended-release performance by first in human studies.
References
- Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
- ICH Q9: Quality Risk Management. International Council for Harmonisation (ICH); 2005.
- Colombo P, Bettini R, Peppas NA. Observation of swelling process and diffusion front position during swelling in HPMC matrices. J Control Release. 2004;97(2):239–47.
- Sun D. Strategies to enhance oral absorption of poorly water-soluble drugs. J Bioequivalence and Bioavailability. 2010;2(2):28–36.
- Kendre PN, Chaudhari SP, Kendre MP, Havaldar VD. Role of acidifiers in solid oral dosage forms: a review. Int J Pharm Sci Res. 2013;4(7):2497–505.
- U.S. Food and Drug Administration. Guidance for Industry: Extended-Release Oral Dosage Forms—Development, Evaluation, and Application of In Vitro/In Vivo Correlations. FDA; 1997.
About The Author:
 Dileep Boinipally is an accomplished pharmaceutical scientist with more than 14 years of hands-on experience in advancing drug products from concept to clinic. He has deep expertise in formulation science and manufacturing process development, particularly for oral therapeutics. Currently, he serves as director of drug product formulation at Metsera, Inc., where he leads formulation development for the company’s oral drug pipeline.
Dileep Boinipally is an accomplished pharmaceutical scientist with more than 14 years of hands-on experience in advancing drug products from concept to clinic. He has deep expertise in formulation science and manufacturing process development, particularly for oral therapeutics. Currently, he serves as director of drug product formulation at Metsera, Inc., where he leads formulation development for the company’s oral drug pipeline.
