mRNA Empowering Engineered In Vivo Cell Therapies
By Deborah Day Barbara, The Alliance for mRNA Medicines; and Joseph Moss and Langlin Cao, Hanson Wade Intelligence

CAR-T therapies experienced a groundbreaking moment in the treatment of patients with pediatric ALL in 2017 upon the approval of Novartis’ Kymriah, the first approved CAR T cell therapy. The overall remission rate was 82.5%, with 63% of patients achieving complete remission. Since that time, six CAR-T therapies have been approved by the FDA, demonstrating transformational success in the treatment of hematologic and lymphatic cancers. Yet, success in the treatment of solid tumors has evaded this modality. All of these regulatory approved CAR-T therapies were developed based on viral vector delivery of the chimeric antigen receptor to create the active pharmaceutical ingredient — genetically modified T cells expressing a molecule on their surface that targets cancer cells. 1
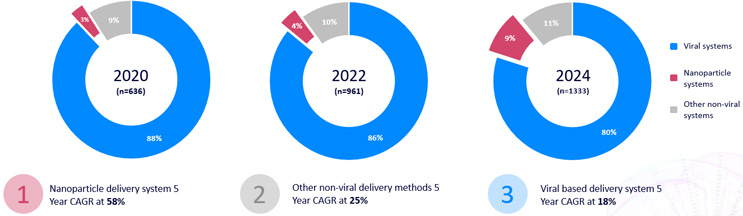
Using mRNA to encode the genes for expression of a CAR in a therapeutic cell is not a new technology. For over a decade, innovators have used a variety of delivery platforms to safely deliver mRNA encoding chimeric antigen receptors ex vivo into T cells to reduce time to patient, mitigate potential safety issues related to viral vectors, and minimize production costs, especially related to analytical testing of viral vectors. These non-viral platforms primarily include PiggyBac transposons, electroporation (including pulsed approaches), and lipid nanoparticles. From 2020 to 2024, there has been a significant increase in popularity in nanoparticles being used as delivery vehicles in CAR cell therapy programs entering the pipeline; these approaches account for ~50% of non-viral approaches used in 2024.
Figure 1: Delivery system landscape and growth for CAR therapeutics, including in vivo and ex vivo therapies. Source: Beacon database. Click on image to enlarge.
In parallel, there has been a movement from almost exclusively genetically modified T cells to a more diverse set of immune cells including macrophages and NK cells. Whereas T cells make up >90% of CAR cell therapies at the clinical stage, a quarter of assets in preclinical development are distinct cell types.
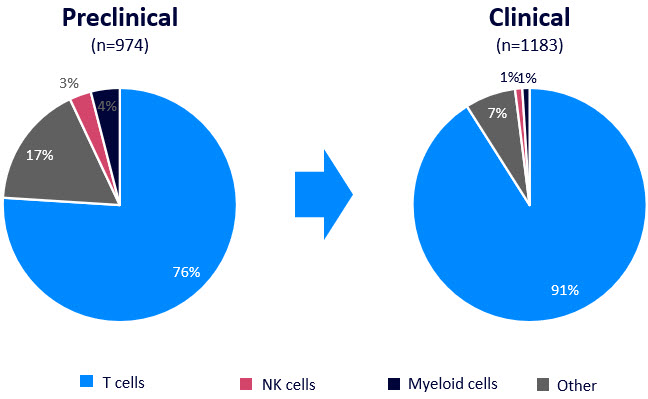
Figure 2: Cell type landscape for CAR therapeutics, including in vivo and ex vivo therapies. Source: Beacon database
These emerging cell types are already being combined with non-viral RNA technologies. An exciting example of this is Moderna’s collaboration with Carisma Therapeutics to develop in vivo CAR-macrophage therapies for the treatment of autoimmune diseases, leveraging Moderna’s mRNA/LNP platform. Preclinical data from the collaboration shows promising results for the therapy in models of metastatic solid tumors. The technology effectively redirects myeloid cells to attack tumor-associated antigens, resulting in strong, targeted anti-tumor activity. This innovative approach offers a ready-made solution that could make CAR-based therapies more accessible.
Advantages Of mRNA-Based Engineered Cell Therapies
Recent advances in non-viral delivery technologies have enabled a first generation of genetically modified immune cells in vivo, including CAR-T, -M, and -NK. Deploying these new approaches has the potential to address several challenges associated with traditional ex vivo CAR-T cell therapy, including:2
- Manufacturing Complexity: Traditional CAR T cell therapies require the extraction of a patient's T cells, genetic modification in a laboratory, and reinfusion back into the patient. This process is time-consuming, expensive, and requires specialized facilities. In vivo mRNA delivery simplifies this by directly delivering the genetic material to the patient's cells, bypassing the need for ex vivo manipulation.
- Logistical Challenges: The logistics of transporting modified T cells from the manufacturing site to the treatment center can be complex and time-sensitive. In vivo delivery eliminates this need, as the genetic material is introduced directly into the patient's body.
- Scalability: Scaling up traditional CAR T cell therapies to treat a large number of patients is challenging due to the personalized nature of the treatment. In vivo mRNA delivery offers a more scalable approach, as it can be standardized and produced in larger quantities.
- Side Effects: In vivo delivery can potentially reduce some of the side effects associated with traditional CAR T cell therapies, such as cytokine release syndrome (CRS) and neurotoxicity, by allowing for more controlled expression of the chimeric antigen receptor.
- Enabling Re-dosing: The transient nature of mRNA expression may allow for re-dosing; conversely, this is not considered as viable with viral approaches due to inherent safety concerns.
Ahhed Tajmohamed, market analyst at Hanson Wade Intelligence, comments, “As the immune cell therapy field searches for safer and more practical delivery system alternatives, LNP is clearly a potentially disruptive technology. As well as enabling multiple sequential gene edits in an ex vivo context, the transient production of T cells in vivo circumvents many of the issues the field still faces today. As the first programs enter the clinic, we need to see if the efficacy of transient CAR T cells can rival the well-established ex vivo viral programs.”
Market Overview Of In vivo Cell Therapies

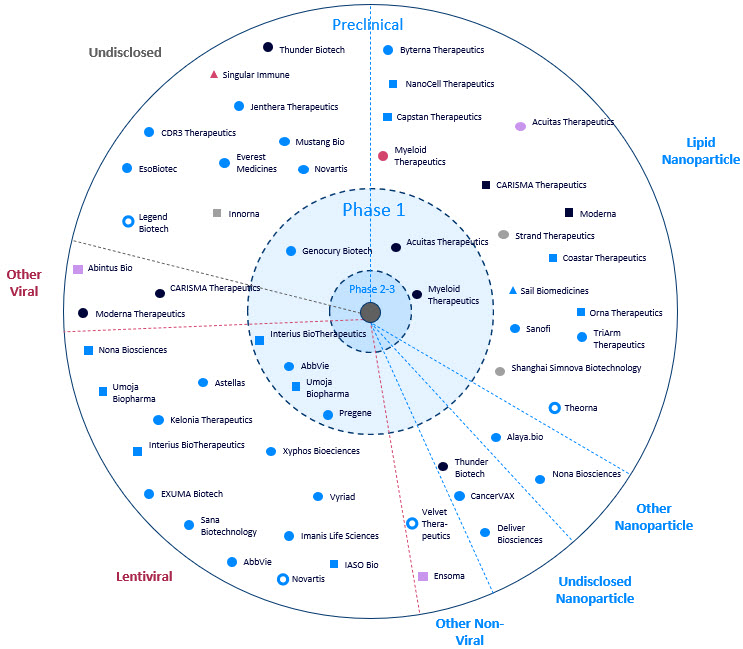
Figure 3: Overview of developers of in vivo CAR programs, including program delivery system, cell type targeted, and indication targeted. Source: Beacon database. Click on image to enlarge.
Capstan Therapeutics recently announced an oversubscribed Series B, signaling the market’s enthusiasm over the transition from ex vivo viral delivery of the CAR to the in vivo non-viral delivery of the CAR.3 It is not just small biotechs exploring in vivo CARs. Sanofi announced during its 2023 R&D Day it has three in vivo CAR-Ts in discovery for treatment of cancer4 and, more recently at the American Society for Hematology meeting, presented a poster that provided preclinical evidence of a high transfection efficiency LNP system for in vivo generation of CAR T cells without systemic toxicities presenting anti-tumor activity.5
The in vivo CAR pipeline is emerging, with 66 assets. While most of the assets are in preclinical development, several assets have entered the clinic for the treatment of solid tumors including colon, lung, liver, breast, and glioblastoma. Early clinical results reported during the November 2024Society for Immunotherapy of Cancer conference on MT-303, an in vivo GPC3-targeting mRNA CAR for advanced liver cancer, showed that this off-the-shelf therapy does not require preconditioning and revealed a favorable safety profile and signs of clinical activity, supporting further development.6
Despite the many challenges associated with viral vectors, particularly safety, they continue to be a viable option for gene delivery. There are currently 23 LV-based in vivo CAR-T therapies under clinical investigation, aimed at assessing their safety and efficacy. These LV-based gene delivery in vivo CAR approaches offer several advantages, such as high transduction efficiency and stable gene expression, which make them an attractive option for certain applications.
However, mRNA delivered by non-viral delivery approaches have gained preference in the in vivo CAR platform landscape. Among these, lipid nanoparticles (LNPs) stand out due to their low immunogenicity, which reduces the risk of an immune response against the delivery system. Additionally, LNPs offer flexibility in terms of mRNA payload delivery and are highly scalable, making them suitable for large-scale manufacturing. This dominance of mRNA-LNPs in the in vivo CAR landscape highlights their potential to provide a safer and more efficient alternative to viral vectors.
CAR-T therapies revolutionized cancer treatment with Novartis’ Kymriah in 2017, marking a breakthrough for pediatric ALL. While FDA approvals have expanded CAR-T success in blood cancers, solid tumors and allogeneic solutions remain challenging. mRNA technology is emerging as a game changer, offering efficient, scalable, and safer options for gene delivery without the complexities of viral vectors. Exciting advancements, like Sanofi’s mRNA/LNP platform, promise to enhance treatment for patient accessibility and effectiveness. This innovative approach is crucial as it aims to simplify manufacturing, reduce side effects, and potentially transform the treatment landscape for solid tumors and other non-oncology diseases.
References
- https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1411393/ful
- https://link.springer.com/article/10.1007/s00005-023-00683-y
- https://www.capstantx.com/posts/capstan-therapeutics-announces-175m-oversubscribed-series-b-financing/#js-refuse-cookies
- https://www.sanofi.com/assets/dotcom/content-app/events/investor-presentation/2023/r-and-d-day-2023/Presentation.pdf
- https://ash.confex.com/ash/2024/webprogram/Paper207566.html
- https://myeloidtx.com/myeloid-therapeutics-announces-sitc-2024-oral-presentation-on-mt-303-the-first-in-vivo-gpc3-targeting-mrna-car-in-human-studies-for-advanced-hepatocellular-carcinoma-hcc/
About the Authors:
 Deborah (Deb) Day Barbara is a cofounder of the Alliance for mRNA Medicines (AMM) and the current chairperson for AMM’s sister organization, the Foundation for mRNA Medicine. A seasoned professional in the life sciences industry, she recently held the position of vice president of strategy & business development at Maravai LifeSciences Holdings, Inc. Prior to Maravai, she held leadership roles at ThermoFisher Scientific, Strategic Diagnostics, GeneLogic, the Johns Hopkins University, and Amersham Life Sciences. With over 30 years of experience, she has made significant contributions to the life sciences industry, particularly in the areas of strategic planning, business development, licensing, intellectual property management, and technology development. Barbara advocates for the advancement of mRNA medicine through the public education strategy initiatives spearheaded by the Foundation.
Deborah (Deb) Day Barbara is a cofounder of the Alliance for mRNA Medicines (AMM) and the current chairperson for AMM’s sister organization, the Foundation for mRNA Medicine. A seasoned professional in the life sciences industry, she recently held the position of vice president of strategy & business development at Maravai LifeSciences Holdings, Inc. Prior to Maravai, she held leadership roles at ThermoFisher Scientific, Strategic Diagnostics, GeneLogic, the Johns Hopkins University, and Amersham Life Sciences. With over 30 years of experience, she has made significant contributions to the life sciences industry, particularly in the areas of strategic planning, business development, licensing, intellectual property management, and technology development. Barbara advocates for the advancement of mRNA medicine through the public education strategy initiatives spearheaded by the Foundation.
 Joseph (Joe) Moss is a senior market analyst at Hanson Wade Intelligence, a market research and consulting firm serving clients in the biopharma industry. He has applied his research experience in biochemistry to strategic research projects in the CAR-T and RNA fields, primarily focusing on emerging technologies. Moss has led several successful research projects in the mRNA-CAR field, contributing to the strategic decisions that may increase access for patients.
Joseph (Joe) Moss is a senior market analyst at Hanson Wade Intelligence, a market research and consulting firm serving clients in the biopharma industry. He has applied his research experience in biochemistry to strategic research projects in the CAR-T and RNA fields, primarily focusing on emerging technologies. Moss has led several successful research projects in the mRNA-CAR field, contributing to the strategic decisions that may increase access for patients.
 Langlin Cao is the product manager at Hanson Wade Intelligence, a market research and consulting firm serving clients in the life sciences industry. Drawing on his background in molecular biology, he began his career as a market analyst, with a specific interest in the cell therapy space. Currently, Cao is focusing on forging connections and facilitating engagements to support the life sciences industry’s efforts to make better drugs faster.
Langlin Cao is the product manager at Hanson Wade Intelligence, a market research and consulting firm serving clients in the life sciences industry. Drawing on his background in molecular biology, he began his career as a market analyst, with a specific interest in the cell therapy space. Currently, Cao is focusing on forging connections and facilitating engagements to support the life sciences industry’s efforts to make better drugs faster.