Preclinical Swine Model Of Large Volume Subcutaneous Injection Pressure Predictive And Translational To Clinical Human Model
By Wendy D Woodley, BS, Didier R Morel, PhD, Diane E Sutter, cCRA, Christopher A. Basciano, PhD, Suvadra S Gerth, BS, Douglas B Sherman, PhD, LuWanda C Chandler, BS, Benjamin L Selvage, BS, Nicholas A Oberlander, Ronald J Pettis, PhD, Natasha G Bolick, MS
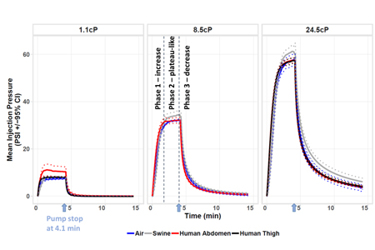
The transition of traditional intravenous in-clinic chronic disease therapies to large volume subcutaneous (SC) injections in alternate settings, i.e. home delivery,1-3,7 has created an evolving and increasing roster of new formulations and treatment options that require the design of innovative, intuitive self-injection drug delivery systems. Characterization of a broad range of applicable injection factors (volume, viscosity, rheological properties) and their impact on biomechanical system function such as fluid path hydrodynamics and injection pressure is vital to drive delivery system design.2-3,5,7
The preclinical swine model is anatomically and physiologically similar to human dermis and subcutis1-3,5-7 (i.e. tissue structure, elasticity, vasculature, absorption characteristics) and enables cost-effective assessment across variable injection conditions for optimizing delivery system and clinical trial designs.
Pump-driven, constant rate 5ml, 1-24.5cP injections were administered into 3 mediums (air, swine and human). Comparison of the injection pressure profiles and characterization of the unique fluid path hydrodynamics4 enabled development of predictive in silico calculations for varying injectate rheological properties. Prior comparison of the corresponding injection tissue effects7 demonstrated parity between the human and swine models and the value of translatable preclinical procedures as a predictive model of system and clinical design.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Drug Delivery Leader? Subscribe today.
