The Path To Commercialization For Wearable Drug Delivery Devices
By Atul Patel, Vice-President, Devices & Delivery Systems, West Pharmaceutical Services
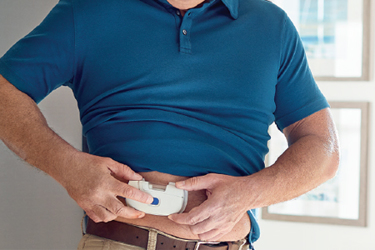
Wearable medical devices have transformed healthcare. Ever since the US FDA granted Cygnus (ID, US) approval to market for its GlucoWatch Biographer as a prescription device for adults with diabetes in 2001,1 the wearables market has continued to evolve and now includes a variety of wearable injectors – devices designed to deliver drugs in large volumes subcutaneously.
Some analysts predict that the global wearable injectors market will reach US$18.3 billion (£13.2 billion) by 2028.2 This growth is being driven by the benefits these devices deliver to various stakeholders. For pharmaceutical and biopharmaceutical companies, wearables offer the opportunity to grow market share and reduce competition. For payers and providers, wearables can reduce costs associated with in-clinic treatment and, potentially, improve outcomes due to better medication adherence. And for patients, wearables offer the convenience of taking medication at home, avoiding the time and effort of visiting a clinic, thereby improving quality of life.
In this article, Atul Patel, Vice-President, Devices & Delivery Systems at West Pharmaceutical Services, looks at the evolution of the wearable drug delivery market, including the route to commercialisation and the challenges faced.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Drug Delivery Leader? Subscribe today.
